Related to Ebola Zaire, Marburg virus disease is fatal in up to 88% of cases, which is why it’s an R&D priority for Sabin.

Marburg FAQ
What is Marburg virus disease?
Marburg virus disease is a hemorrhagic fever
virus that can disrupt the body’s clotting system and lead to internal bleeding. It is in the same family as the virus that causes Ebola virus disease and has a high case fatality rate of 25-88%.
How quickly does a person experience symptoms after infection?
Symptoms develop between two to 21 days after transmission, depending on the route of infection and infectious dose.
What are the symptoms?
Symptoms begin abruptly with a high fever, acute headache, and extreme fatigue. As the disease progresses, it becomes severe and can include pancreatic inflammation, shock, liver and immune system failure, and massive hemorrhaging.
How does a person get infected with Marburg?
Marburg is most often transmitted upon extremely close contact with an infected or deceased person, usually via contact with blood, saliva, urine, or other bodily fluids. Contact with contaminated clothing, bedding and medical equipment are other channels of transmission.
Where have cases of Marburg been found?
The first known outbreaks of Marburg occurred in 1967 in Germany and Serbia. Since then, most outbreaks have occurred in Sub-Saharan Africa, but it has spread to other parts of the world in isolated instances.
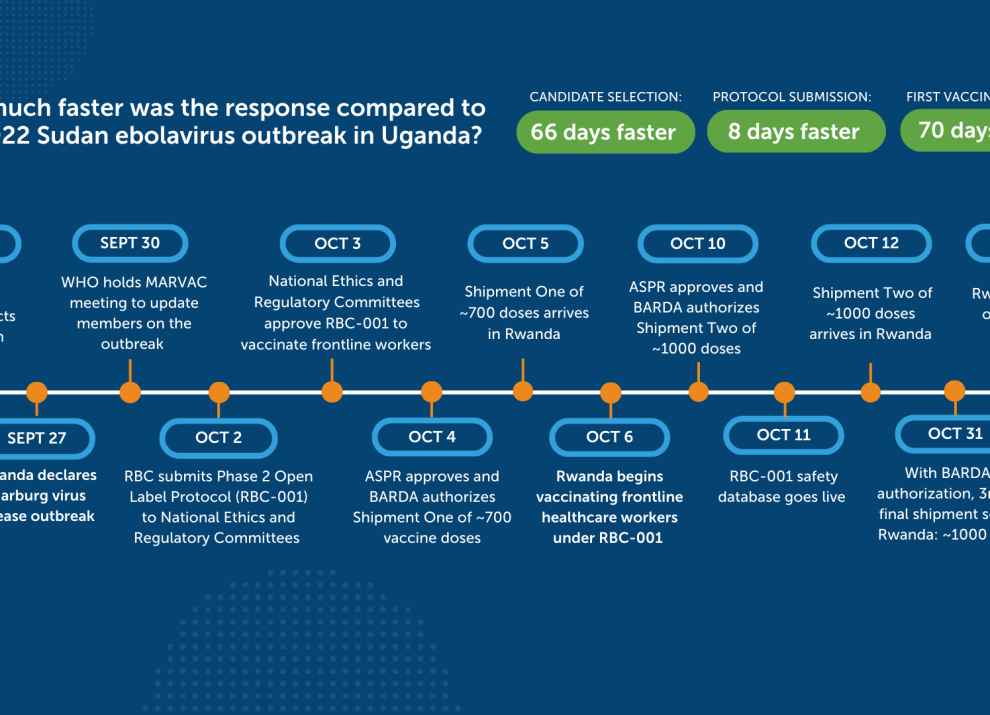
When was the last outbreak of Marburg?
In September 2024, an outbreak of Marburg was declared in Rwanda, In 2023, Tanzania and Equatorial Guinea both experienced Marburg outbreaks.
Is there a treatment or vaccine for Marburg?
There is no proven treatment for Marburg virus disease, but potential treatments including blood products, immune therapies, and drug therapies are currently in development. There is also currently no approved vaccine for Marburg, but Sabin currently has a candidate in Phase 2 clinical trials.
What is the status of Sabin’s Marburg vaccine candidate?
Since October 2023, Sabin is running a Phase 2 clinical trial for its vaccine candidate against the Marburg virus. Healthy adult volunteers have received the single-dose vaccine candidate at Makerere University Walter Reed Project in Kampala, Uganda, and at the Kenya Medical Research Institute in Siaya, Kenya.
The Fatality of Marburg


We make vaccines more accessible, enable innovation and expand immunization across the globe.