Powering Vaccine R&D

About Our Vaccine R&D Program
Our Research & Development program prioritizes strong vaccine candidates for infectious diseases that lack commercial interest and disproportionately affect the world’s most vulnerable populations. Current efforts include developing vaccines for Marburg and Ebola Sudan viruses.

Vaccine Development for Marburg Virus Disease
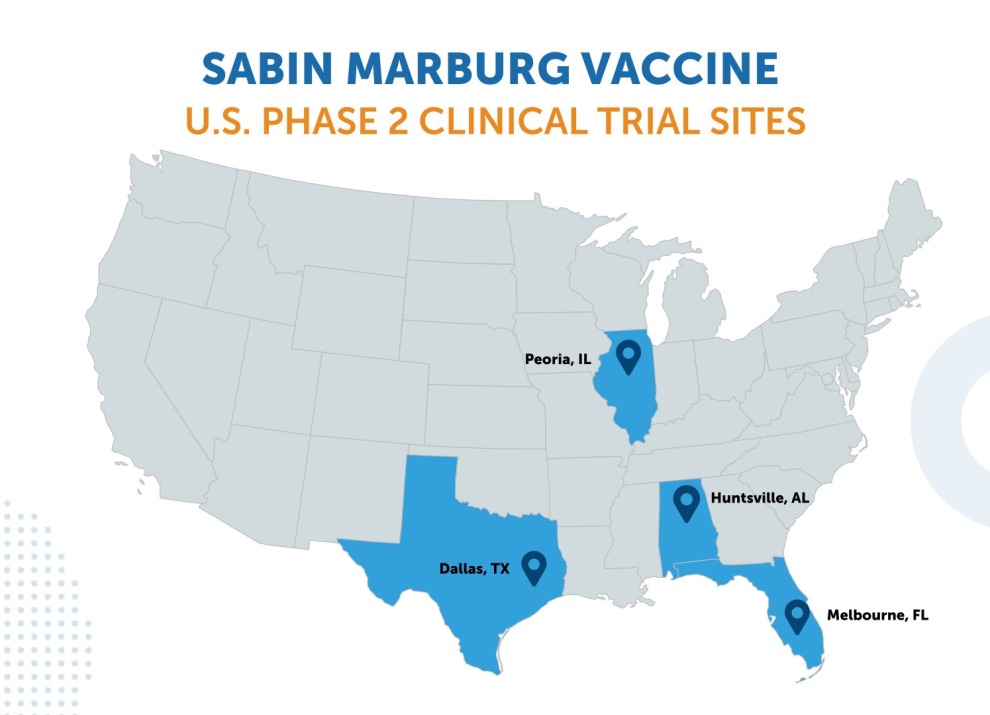
Marburg virus disease is a serious and often deadly illness caused by a filovirus — the same family that also includes Ebola. With no licensed vaccine currently available, Sabin’s R&D program is advancing a chimpanzee adenovirus type 3 (cAd3) vaccine to protect against this disease threat. Fatality rates for Marburg have ranged from 24 to 88% in past outbreaks. A Phase 2 clinical trial for Sabin’s Marburg vaccine candidate is currently underway.
Vaccine Development for Sudan Ebolavirus Disease
Sudan ebolavirus disease is a severe and often fatal illness caused by a filovirus, the same family as the Marburg virus. Since no licensed vaccine exists, Sabin’s R&D program is developing a chimpanzee-adenovirus type 3 (cAd3) vaccine to help protect against this deadly pathogen. Fatality rates for Sudan virus disease have varied from 41% to 100% in past outbreaks. A Phase 2 clinical trial for Sabin’s Sudan vaccine candidate is now underway.

Outbreak Response
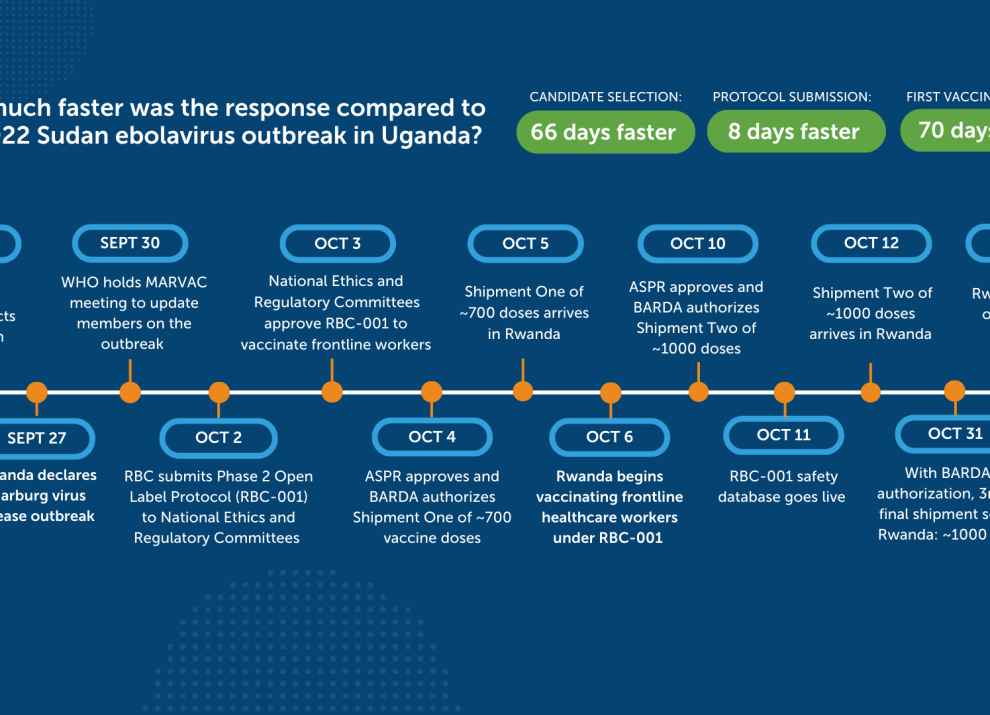
Marburg and Sudan ebolavirus are among the world’s deadliest diseases, with increasing outbreaks and high fatality rates—claiming, on average, one in two lives. In recent years, Sabin has supported outbreak responses for both viruses, including delivering investigational vaccine to Rwanda in 2024.

Resources
Explore a curated collection of research on Marburg and Sudan ebolavirus vaccine investigations, including trial information, journal studies, and findings from Sabin’s ongoing efforts to combat these deadly diseases.

Clinical Partnerships
Sabin’s rigorous research is supported by scientific partners. To articulate our clinical research partnership philosophy, Sabin created a Framework that is designed to provide transparency and guidance as we work with partners now and in the future to advance vaccine candidates through the clinical development and regulatory processes with the goals of licensure and WHO prequalification.
R&D Support
Sabin’s Marburg and Sudan ebolavirus vaccine development builds on research performed previously by the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center and GSK. This work is currently funded under multi-year contracts with the Biomedical Advanced Research and Development Authority (BARDA), a part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services. As of January 2025, Sabin has received just over $250 million in contract awards from BARDA for furthering vaccine research and development against these two disease threats.
Vaccine Platform
In August 2019, Sabin announced exclusive agreements with GSK for Sabin to advance the development of the prophylactic candidate vaccines against the deadly Ebola Zaire, Sudan ebolavirus and Marburg virus. Under the agreements between Sabin and GSK, Sabin exclusively licensed the technology for all three candidate vaccines and acquired certain patent rights specific to these vaccines. The three candidate vaccines — initially developed collaboratively by the U.S. National Institute of Allergy and Infectious Diseases’ Vaccine Research Center and Okairos (acquired by GSK in 2013) — are based on GSK’s proprietary chimpanzee-adenovirus type 3 (cAd3) platform. Sabin has since advanced the development of the Marburg and Sudan ebolavirus vaccines through Phase 2 trials, as no licensed vaccines exist for these viruses. Sabin did not pursue research on Ebola Zaire, as an approved vaccine already exists for this disease.

We make vaccines more accessible, enable innovation and expand immunization across the globe.