Local Research Could Curb Cholera at Global Scale
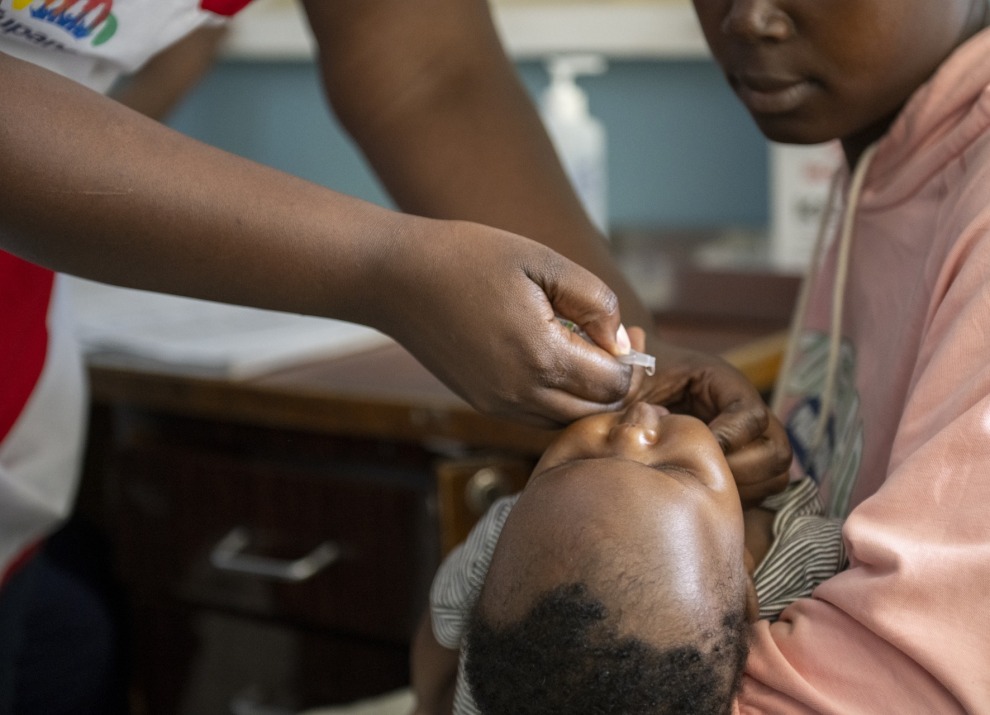
In late 2024, the Wellcome Trust awarded Sabin’s Applied Epidemiology team — in partnership with Washington State University (WSU), Massachusetts General Hospital, and researchers from the Kenya Medical Research Institute (KEMRI) — with funding to generate critical data on the use of the oral cholera vaccine (OCV) with the goal of informing cholera vaccination strategies in Kenya and other endemic regions. Worldwide cases of cholera have soared over recent years — with a 71% increase in deaths in 2023 over 2022 numbers — due to several factors including unprecedented weather events resulting from climate change, as well as conflict and displacement.
The project will take place in Nairobi, with some activities happening in the informal settlement of Mukuru — a hotspot for cholera given its crowded living conditions and less-than-ideal water and sanitation infrastructure. The team will assess immunogenicity of the Euvichol-S OCV through a randomized controlled trial and will also analyze the effectiveness of Kenya’s first-ever preventative vaccination campaign for cholera.

Prequalified by the World Health Organization in April 2024, Euvichol-S is a simplified formulation of existing OCVs. This newer product should allow for rapid, increased production capacity in times of need. Given the current low supply of non-emergency cholera vaccines in conjunction with the increasing number of outbreaks worldwide, Euvichol-S could be a game-changer in the fight against the disease at subnational, national, and global levels.
For the randomized trial, the team will look at the time between two doses of OCV and how the immune system reacts if doses are given two weeks apart, per current recommendations for Euvichol-S administration, or three months, or one year. The trial includes different age groups — including children under 5 years old — to compare age-specific responses.
Findings from the trial can show the best way to protect the most vulnerable population from dying from cholera — young children. One-third of the cholera cases reported during Kenya’s most recent outbreak (between October 2022 and February 2024) were among children under 10 years of age. Research on optimal cholera vaccine schedules for children one to five years is ranked by the Global Task Force for Cholera Control (GTFCC) as the highest priority in the Cholera Roadmap Research Agenda.
To determine the effectiveness of the Kenyan vaccination campaign, the team will analyze data from different sub-counties in Nairobi on vaccination coverage, case trends, and changes within and between various geographic areas to see how the campaign changes how many people get sick with cholera. They will also look at the indirect protection of the vaccine: how much living near other vaccinated people reduces your risk of getting sick.
 Sabin Vice President for Applied Epidemiology Dr. Denise Garrett visited Mukuru and her colleagues at KEMRI and WSU late last year. Below she shares some of the reasons behind the project and its potential outcomes.
Sabin Vice President for Applied Epidemiology Dr. Denise Garrett visited Mukuru and her colleagues at KEMRI and WSU late last year. Below she shares some of the reasons behind the project and its potential outcomes.
Can you describe the need for this work in Mukuru and similar communities?
During Kenya’s most recent cholera outbreak, this community was hit hard. The research we are doing has the potential to change the lives of those living there and improve outcomes for so many living in similar conditions around the world. It’s only logical that it should be conducted in a place that can benefit the most.
What are some of the challenges that partners on the ground will face as they begin this work?
This study is complex and requires complicated logistics to enroll and follow a large number of participants. We are so grateful to be working with experienced collaborators with a long history of work in Mukuru who are excited to contribute to an important research base that can benefit the community there and others around the country.
How on track are we to achieve the GTFCC’s goal to reduce cholera deaths by 90% and eliminate the disease in 20 countries by 2030?
Without sustained effort and investment, we will not be able to reach this goal. Despite progress in policies, vaccination campaigns, and technical guidance, there are acute funding needs to address both treating cholera disease in outbreak and endemic settings as well as the upstream factors that lead to cholera, like water and sanitation.
How will this work contribute to achieving that goal?
This work will inform how we can best prevent cholera through vaccines overall. We expect to also have results on how the body responds, immunologically, to the cholera vaccine and how community disease burdens respond to vaccination campaigns. Combining the trial results and the analysis of case data will provide critical evidence and relevant insights to inform cholera vaccination strategies, optimize vaccine utilization, and strengthen cholera control efforts in Kenya and beyond.
The project will span multiple years, with initial findings expected in 2027.
Recommended for you

We make vaccines more accessible, enable innovation and expand immunization across the globe.





